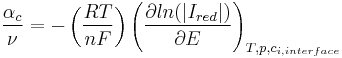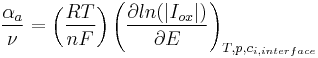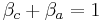Charge transfer coefficient
Charge transfer coefficient, and symmetry factor (symbols α and β, respectively) are two related parameters used in description of the kinetics of electrochemical reactions. They appear in the Butler-Volmer equation and related expressions.
The symmetry factor and the charge transfer coefficient are dimensionless.[1]
Contents |
Charge transfer coefficient
According to an IUPAC definition[2], for a reaction with a single rate-determining step, the charge transfer coefficient for a cathodic reaction (the cathodic transfer coefficient, αc) is defined as:
The anodic transfer coefficient (αa) is defined by analogy:[3]:
where:
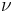 : stoichometric number, i.e., the number of activated complexes formed and destroyed in the overall reaction (with n electrons)
: stoichometric number, i.e., the number of activated complexes formed and destroyed in the overall reaction (with n electrons)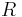 : universal gas constant
: universal gas constant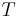 : absolute temperature
: absolute temperature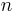 : number of electrons involved in the electrode reaction
: number of electrons involved in the electrode reaction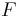 : Faraday constant
: Faraday constant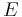 : electrode potential
: electrode potential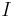 : partial cathodic (anodic) current[4][5]
: partial cathodic (anodic) current[4][5]
Interpretation
The charge transfer coefficient signifies the fraction of the interfacial potential at an electrode-electrolyte interface that helps in lowering the free energy barrier for the electrochemical reaction. The electroactive ion present in the interfacial region experiences the interfacial potential and electrostatic work in done on the ion by a part of the interfacial electric field. It is charge transfer coefficient that signifies this part that is utilized in activating the ion to the top of the free energy barrier.
Batteries and fuel cells
In operating batteries and fuel cells, charge transfer coefficient is the parameter that signifies the fraction of overpotential that affects the current density. This parameter has had a mysterious significance in electrochemical kinetics for over three quarters of the previous century. It can also be said that charge transfer coefficient is the heart of electrode kinetics.
Symmetry factor
The symmetry factor (or barrier symmetry factor) is a coefficient similar to the transfer coefficient, but applicable only to a single-step reactions.
The sum of anodic symmetry factor and cathodic symmetry factor are equal to one:
Interpretation
References
- ^ IUPAC. "Quantities, Units and Symbols in Physical Chemistry" ("The Green Book"), 2nd edition, Blackwell Science, 1993. (on line)
- ^ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. doi:10.1351/goldbook. Entry: "Cathodic transfer coefficient".
- ^ http://goldbook.iupac.org/A00371.html
- ^ http://goldbook.iupac.org/P04407.html
- ^ IUPAC. ELECTRODE REACTION ORDERS, TRANSFER COEFFICIENTS AND RATE CONSTANTS. APPLICATION OF DEFINITIONS AND RECOMMENDATIONS FOR PUBLICATION OF PARAMETERS. Pure & Appi. Chern., Vol. 52, pp.233—240, 1979. (pdf)